Zinc Plating and Galvanised Protective Layers
 Zinc surface layers on steel, whether electroplated or hot dipped galvanised, are applied to provide corrosion protection in atmospheric environments by forming a protective zinc carbonate film when dry and acting as a sacrificial anode where damaged. The thicker the zinc layer the longer it will provide protection.
Zinc surface layers on steel, whether electroplated or hot dipped galvanised, are applied to provide corrosion protection in atmospheric environments by forming a protective zinc carbonate film when dry and acting as a sacrificial anode where damaged. The thicker the zinc layer the longer it will provide protection.
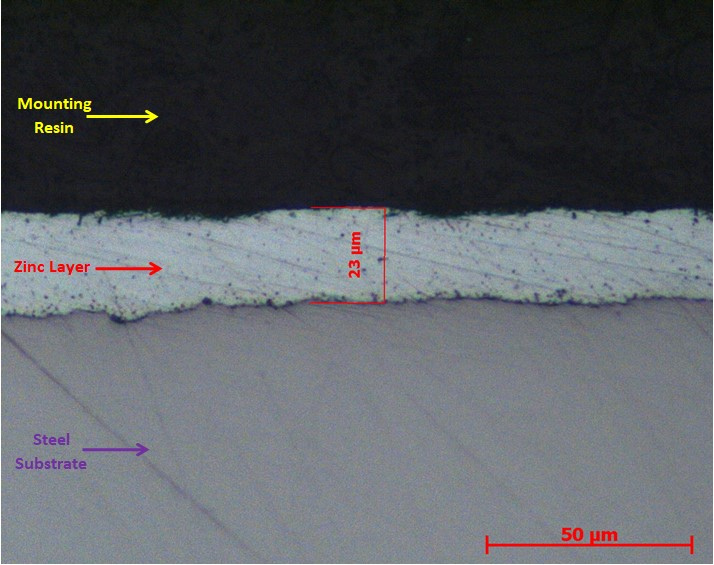 Generally the zinc layer is specified as a minimum mass per surface area. Clients cannot always verify whether the galvanising conforms to the specified mass per area criteria. There is, however, a direct relationship between the mass per area and the thickness. LPD Lab Services can measure the thickness of the zinc layer on a cross section of the material and, from that, estimate the mass per area, as per BS EN ISO1461.
Generally the zinc layer is specified as a minimum mass per surface area. Clients cannot always verify whether the galvanising conforms to the specified mass per area criteria. There is, however, a direct relationship between the mass per area and the thickness. LPD Lab Services can measure the thickness of the zinc layer on a cross section of the material and, from that, estimate the mass per area, as per BS EN ISO1461.
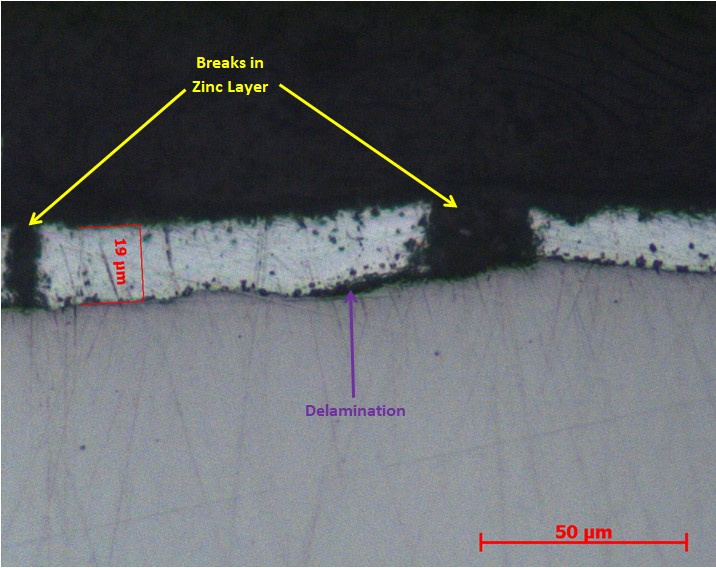
LPD Lab Services can also analyse coating failures which might typically be due to improper surface preparation, inadequate coating or insufficient coating thickness.
Hot dipped zinc layers are much thicker than electroplated zinc layers and will always greatly outlast zinc electroplating in any environment. Electroplated zinc coatings are, therefore, rarely adequate for outdoor use.
 Electroplated zinc is susceptible to the formation of zinc whiskers, but hot dipped coatings are much less susceptible. If electroplated zinc products are used in a hospital or computer room there is the risk that zinc whiskers can cause misfortune. LPD Lab Services has the experience and capability to identify and quantify zinc whiskers using scanning electron microscopy SEM.
Electroplated zinc is susceptible to the formation of zinc whiskers, but hot dipped coatings are much less susceptible. If electroplated zinc products are used in a hospital or computer room there is the risk that zinc whiskers can cause misfortune. LPD Lab Services has the experience and capability to identify and quantify zinc whiskers using scanning electron microscopy SEM.

