Pharmaceutical and Filler Particle Counting
The particle and size distribution characteristics of a pharmaceutical drug often have an impact on the rate of delivery in the body. The regulatory bodies (e.g. FDA) have a stringent requirement of understanding the chemical and physical characteristics of drug particles and potential contaminants as part of the drug validation process and this can be achieved using SEM/EDX Automated Particle Characterisation and Analysis.
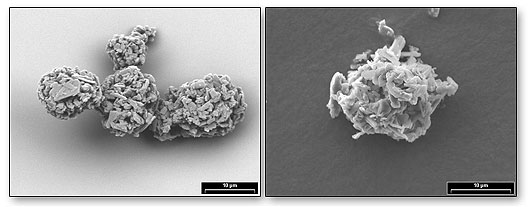 |
| SEM images of respirable asthma drug particles |
Historically, optical light microscopy has been sufficient, however more recently the regulatory authorities are increasingly insisting on characterising finer particles and agglomerates unresolvable in transmission or reflection by optical microscopy. This shortcoming can be overcome by using Scanning Electron Microscopy SEM.
Characterisation of Smaller Drug Particles
The SEM, coupled with the unique combination of staff experience and software, can automatically perform analysis down to a particle size of 2µm. Below this manual operation is required (ultimate resolution of SEM is 2 nm) with the advantage of chemical characterisation using EDX. The field of view is determined by the particle size range to be analysed.
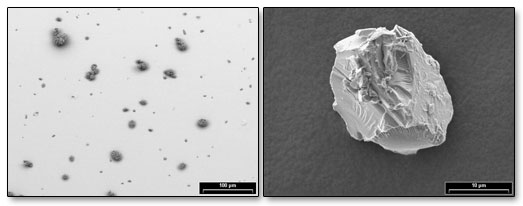 |
| SEM images showing typical particle distribution and contaminant fine glass particle |
 If multiple fields are to be analysed, as is commonplace within the pharmaceutical industry, a matrix of areas needs to be defined like that shown below.
If multiple fields are to be analysed, as is commonplace within the pharmaceutical industry, a matrix of areas needs to be defined like that shown below.
Within each field of view, the particles can be automatically characterised by Automated SEM/EDX according to one or more of the following parameters:
- Chemical composition by EDX
- Dimensional Characteristics
- Total Area
- Shape Factor
- Size
- Diameter
- Aspect Ratio
- Coordinates.
 An example of the data available is shown to the right:
An example of the data available is shown to the right:
The SEM/EDX Automated Particle Characterisation and Analysis technique allows for the identification of the critical characteristics of particles. It offers the ability to gather information about finer particles than by optical microscopy and can readily distinguish between clusters and agglomerates of particles in addition to the chemical analysis available by EDX.
The strength of this automated analysis technique is its ability to gather statistically significant data on the size, morphology and composition of the particles in a time efficient manner, beyond the capabilities of conventional optical microscopy. Pareto plots can be employed to focus the customer on the most frequently encountered particles in order to eliminate the key contaminants.
Please contact us today to discuss your particle characterisation needs.
Application Notes
| Document Title | View |
| Pharmaceutical Particle Counting, Size Measurement and Chemical Analysis by SEM/EDX | |
| Contaminant Particle Identification and Elimination |

